Where is Chlorhexidine used?
The pharmaceutical product is used for the treatment and prevention of diseases caused by microorganisms sensitive to Chlorhexidine.
Depending on the concentration of the substance, the medicine can be used in the treatment of various pathologies.
After operations, in order to prevent infectious pathologies, doctors resort to using a pharmaceutical solution.
Chlorhexidine is often used for dental purposes for the treatment of dentures. In some cases, medication is used to treat periodontitis, stomatitis, and they are used to rinse the gums.
The medicine is used in:
- Urology
- Gynecology
- Surgery
Medical Internet conferences
Chlorhexidine is an antiseptic that does not lead to bacterioresistance
Pogosyan M.A.
Scientific supervisor: assistant of the Department of Pediatric Dentistry and Orthodontics, Ph.D., Kazakova L.N.
GBOU VPO Saratov State Medical University named after. IN AND. Razumovsky Ministry of Health of the Russian Federation
Department of Pediatric Dentistry and Orthodontics
An analysis of Russian and foreign medical literature has shown that for more than 60 years of active use of chlorhexidine in the clinic, there are no reports of microflora resistance to it.
Chlorhexidine is an amphipathic molecule with hydrophilic and hydrophobic groups and is a cation at physiological pH. The molecule consists of two symmetrical chlorophenol rings (4-chlorophenyl) and two biguanide groups united in the center by a hydrophobic hexamethylene chain, being a symmetrical molecule - 1,6-bi-4-chlorophenyldiguanidohexane [1, 2]. Typically, chlorhexidine is used in the form of salts, mainly biacetate, bigluconate or dihydrochloride [3,4,5]. Bigluconate is most soluble in water and alcohols [5]. In addition, this form has the additional advantage that the active components with a positive ionic charge are released at physiological pH values. Chlorhexidine is practically not absorbed from the gastrointestinal tract. After accidental ingestion, the drug is not detectable in the blood after 12 hours. Due to its cationic nature, chlorhexidine binds well to the skin and mucous membranes. Chlorhexidine is not inactivated by blood and plasma proteins, is distributed locally when applied cutaneously and is practically not involved in metabolism. Chlorhexidine is widely used in medicine as an antiseptic and disinfectant for surfaces and instruments. For diseases in surgery, dentistry, gynecology, urology, dermatovenereology, otolaryngology, it is used locally. It is the main antiseptic for treating the surgical field, surgeon's hands, and medical staff's hands in postoperative care for patients in ENT departments and in cosmetology. Chlorhexidine is characterized by a pronounced antibacterial effect and substance compared to other antiseptics used to treat the oral cavity. [6]. At a dental appointment, chlorhexidine is offered in concentrations of 0.05%; 2% in the form of solutions and Diplen-Denta X, containing chlorhexidine digluconate 0.01-0.03 mg per 1 sq. cm.
What concentration of chlorhexidine has the best properties, which is more effective in the treatment of caries of primary and permanent teeth in children?
Purpose of the work: to compare the effectiveness of chlorhexidine bigluconate at a concentration of 0.05%; 2% in relation to microflora taken from the carious cavity at different depths.
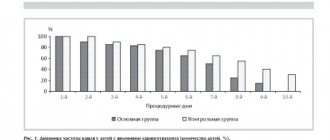
Materials and methods : we conducted a study of the quality of the microflora of 20 carious cavities: 10 in baby teeth and 10 in permanent teeth. Infected dentin of the carious cavity was taken with a sterile bur, bacteriological examination was carried out according to all the rules of clinical microbiology, to isolate aerobic and optionally anaerobic microorganisms. During primary culture, multiple streptococci and staphylococci were isolated from carious cavities and in milk and permanent teeth. Biochemical identification of pure cultures of streptococci, enterococci, and staphylococci was not performed. To identify the sensitivity of microflora to chlorhexidine, the crops on a Petri dish were treated with various concentrations of an antiseptic, and after 1 and 3 days the possible growth was analyzed. In both cases: when using 0.05% and 2% chlorhexidine, no growth of cultures was detected.
Analysis of the results obtained confirms the continued high activity of chlorhexidine against pathogens present in the carious cavity and their lack of resistance. The same final result obtained during the study using different concentrations of the antiseptic allows us to experimentally substantiate the feasibility of using chlorhexidine 0.05% in pediatric dentistry.
Contraindications
Chlorhexidine is contraindicated:
- In case of hypersensitivity to the components of the drug
- People suffering from dermatitis
- Do not use together with other antiseptics, for example, with hydrogen peroxide
- For ophthalmic use, rinsing the eyes with this product is prohibited.
- For disinfection of the surgical field
- After intervention on the auditory canal and central nervous system
It is important to know that the pharmaceutical product should be used with caution when treating children.
Brief instructions for use
To prevent sexually transmitted diseases, after unprotected sexual intercourse, after a maximum of 2 hours, 2-3 ml of a 0.5% solution should be administered into the urinary canal of a man, 1 ml into the canal for women and 5-10 ml into the vagina. You can treat skin areas near the genitals with the product. After administering the drug, try to postpone urination for 2 hours.
Your doctor should tell you about douching for gynecological diseases.
For sore throat, rinse the mouth with 0.5% or 0.2% Chlorohexidine solution.
For inflammatory pathologies of the urinary tract, it is necessary to inject 2-3 ml of 0.05% of the product into the urinary canal.
Before using the drug, you should consult your doctor.
Recently, due to the spread of infections of viral etiology, including respiratory viral diseases, the question arises: does chlorhexidine digluconate (CHB) have antiviral activity?
Chlorhexidine is used in the form of salts, mainly as bigluconate. CHB is most soluble in water and alcohols, and hence this form is used to make antimicrobial agents. CHB is a universal skin antiseptic that kills many pathogenic microorganisms, but is safe for humans. Currently, in clinical practice, CHB is preferred not only when treating the skin (hands, surgical field), but also as an oral antiseptic. The therapeutic activity and safety of CGB have been studied in dozens of international clinical studies. It is characterized by antimicrobial activity against gram-positive and gram-negative bacteria, fungi, and also negatively affects the causative agents of certain viral infections.
Many scientific papers have been published studying the antiviral activity of CGD. At the same time, some sources confirm the virucidal effect of CHB, while others question this effect in relation to some viruses.
Karpinski T.M. and Szkaradkiewicz AK in an article describing a wide range of pharmacological activity of CHB, indicate that under the influence of CHB, complex viruses surrounded by a lipoprotein shell (herpes simplex virus, HIV, influenza virus, cytomegalovirus) are quickly inactivated [1]. Wood A. and Payne D. in their study confirm the inactivating effect of chlorhexidine on herpes simplex virus type 1 and HIV type 1 [2].
In addition, Bernstein D. et al. in a study of the antiviral effectiveness of Peridex mouth rinse (in vitro), containing 0.12% CHB, they proved the virucidal activity of the drug against several viruses: herpes simplex (HSV), cytomegalovirus (CMV), influenza A, parainfluenza and hepatitis B (HBV) ) and the ineffectiveness of the drug against the polio virus [3]. Antiviral activity increased with increasing time of exposure of the drug to the mucous membranes of the oral cavity. Thus, it has been suggested that CGB exerts an antiviral effect by targeting the enveloped herpes simplex, influenza A, parainfluenza, hepatitis B, and cytomegalovirus viruses, but has no effect on the nonenveloped poliovirus.
CHB acts at the level of the cell membrane, increasing its permeability [4]. First of all, it destroys the capsid - the protein shell of the virus.
A new study conducted in 2022 confirmed the susceptibility of the SARS-CoV-2 virus to the effects of a 0.05% CHB solution. Viral culture testing showed that the SARS-CoV-2 virus was undetectable after a 5-minute incubation at room temperature (22°C). This conclusion was reached by scientists from the Medical University of Hong Kong, whose work was published in the scientific journal The Lancet Microbe [5].
Based on the above, we can conclude that CHB has a certain antiviral activity against complex (enveloped) viruses and is currently one of the main antiseptics in the range of drugs for treating the oral cavity, skin, and hands of medical personnel.
- Karpinski T.M., Szkaradkiewicz AK Chlorhexidine — Pharmaco-biological activity and application. European Review for Medical and Pharmacological Sciences, 2015; 19: 1321-1326.
- Wood A, Payne D. The action of three antiseptics/disinfectants against enveloped and non-enveloped viruses. J Hosp Infect 1998; 38: 283-295.
- Bernstein D., Schiff G., Echler G., Prince A., Feller M., Briner W. In vitro virucidal effectiveness of a 0.12%-chlorhexidine gluconate mouthrinse. J Dent Res. 1990 Mar; 69(3):874-6.
- Zverkov A.V., Zuzova A.P. Chlorhexidine: past, present and future of one of the main antiseptics. Scientific and practical journal “Clinical microbiology and antimicrobial chemotherapy”. – Smolensk: Interregional Association of Public Associations “Interregional Association for Clinical Microbiology and Antimicrobial Chemotherapy”, 2013. – T. 15, No. 4, pp. 279-285.
- Chin A., Chu J., Perera M. et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020; published online, April 02, 2022.
Technologist for drug development at Retinoids JSC Krot S.L.
Seal
Where to put candles
Depending on the type of disease, the method of administering Chlorhexidine suppositories can be different: rectal or vaginal.
But it is important to understand that the use of suppositories is only permissible as prescribed by a doctor.
Before using the suppository, you need to wash and dry your hands well, then you need to remove it from the package and insert it into the vagina in a lying position.
To avoid leakage of the drug, do not rush to get out of bed.
Chlorhexidine suppositories help well with thrush and cystitis.
Modern Pharmaceuticals of Russia
Chlorhexidine bigluconate 0.05% 100 ml
APPLICATION
The product is intended for use in medical institutions: hygienic treatment of the hands of medical personnel, including ambulance personnel; cleaning the hands of surgeons, operating nurses, midwives and other persons involved in operations and childbirth; hygienic treatment of the hands of medical personnel of medical institutions, the hands of medical workers of preschool and school institutions, social security institutions.
And also for the hygienic treatment of hands of students in educational institutions;
hygienic treatment of hands of workers of perfumery, cosmetics, chemical and pharmaceutical enterprises, public utility facilities; workers of food enterprises, public catering enterprises and food trade enterprises. Hygienic treatment of hands of workers of poultry, livestock, pig and fur farms; hand treatment for the population, protection from microbes in public places, in nature, and also as a prophylactic agent in the form of irrigation, rinsing and applications on the surface of the skin and mucous membranes. PACKAGING
Packaging is carried out in 25 ml, 50 ml, 100 ml in orange glass bottles with a screw neck, sealed with polyethylene stoppers and screw caps.
25 ml, 50 ml, 100 ml, 200 ml, 500 ml, 1000 ml in high-pressure polyethylene bottles with a polymer nozzle or a special nozzle with a screw cap, or equipped with a spray pump and a protective polyethylene cap.
5 l, 10 l, 20 l in canisters made of low-density polyethylene (for hospitals).
Glass bottles of 25 ml, 50 ml, 100 ml are placed in boxes with partitions or corrugated cardboard bars.
Bottles of 25 ml, 50 ml, 100 ml, 200 ml, 500 ml, 1000 ml made of high-density polyethylene should be packaged in polyethylene shrink film or in a box with partitions or grids made of corrugated cardboard.
Polyethylene canisters are placed in a wooden pallet, no more than 2 rows high, and covered with stretch film.
| Product | Individual box | Individual label | Group label | G/I size | Number of bottles per g/y |
| CGD, 0.05%, 100 ml | — | 45×67 | 93×70 | 360x226x248 with gasket | 80 |
| CGD, 0.05%, 100 ml (blue) | 48x48x130 | 45×67 | 93×70 | 255x255x270 | 50 |
| CGD, 0.05%, 100 ml (pink) | 48x48x130 | 45×67 | 93×70 | 255x255x270 | 50 |
BARCODES
Individual
| CGD, 0.05%, 100 ml | CGD, 0.05%, 100 ml (blue) | CGD, 0.05%, 100 ml (pink) |
| 4620008620039 | 4620008620381 | 4620008620374 |
Group
| CGD, 0.05%, 100 ml | CGD, 0.05%, 100 ml (blue) | CGD, 0.05%, 100 ml (pink) |
| 24620008620033 | 14620008620388 | 14620008620371 |
INSTRUCTIONS for using the disinfectant
Registration number:
RU.77.99.01.002.E.031065.08.11
Trade name of the drug:
Chlorhexidine bigluconate 0.05% – YuzhPharm
International nonproprietary name:
Chlorhexidine
Form:
Colorless or light yellow transparent liquid.
Compound:
chlorhexidine bigluconate, 0.05%, purified water.
Description:
"Chlorhexidine bigluconate 0.05% - YuzhPharm" is a ready-to-use product in the form of a clear or slightly opalescent liquid, colorless or light yellow, odorless or with a faint odor.
Group:
Disinfectant.
PROPERTIES:
The product is bactericidal against gram-positive and gram-negative bacteria (including pathogens of nosocomial infections), tuberculocidal, virucidal (acute respiratory viral infections, herpes, polio, hepatitis of all types, including hepatitis A, B and C, HIV infection, adenovirus etc.) and fungicidal (against fungi of the genera Candida and Trichophyton) action. According to the parameters of acute toxicity, “Chlorhexidine bigluconate 0.05% - YuzhPharm” belongs to class 4 of low-hazardous substances according to GOST 12.1.007-76 when applied to the skin, introduced into the stomach and inhaled. According to the classification of Sidorov K.K. when administered parenterally, the drug belongs to class 5 of practically non-toxic compounds. Skin-resorptive and sensitizing properties in the recommended modes of use of the product have not been identified. The product has a moderate irritating effect on the mucous membranes of the eyes. The maximum permissible concentration in the air of the working area of chlorhexidine bigluconate is 1 mg/m3.
The product can be used to treat the skin of children from birth.
INDICATIONS FOR USE:
The product "Chlorhexidine bigluconate 0.05% - YuzhPharm" is intended for use in medical institutions:
- for hygienic treatment of the hands of medical personnel, including personnel of ambulances;
- for disinfecting the skin of the injection field;
- for sanitary treatment of the skin and the skin of the feet to prevent fungal diseases;
- for disinfection of rubber gloves worn by personnel (made of materials resistant to chemicals) during surgical interventions and manipulations requiring surgical antiseptics, when working with potentially infected material (microbiological and other laboratories); when collecting medical waste of classes B and C.
- for disinfection of small instruments of simple configuration;
- as a prophylactic agent in the form of irrigation, rinsing and application on the surface of the skin, mucous membranes;
- for hygienic treatment of hands of students of general education institutions, staff of preschool and school institutions, social security institutions (nursing homes, hospices, etc.), employees of perfumery and cosmetics enterprises (including hairdressers, beauty salons, etc.), public food, communal facilities, food and chemical-pharmaceutical industries;
- for hygienic treatment of the skin of the hands, injection field, sanitary treatment of the skin and skin of the feet for the purpose of preventing fungal diseases, as a prophylactic for the population in everyday life.
CONTRAINDICATIONS:
Skin-resorptive and sensitizing properties have not been identified in the recommended modes of use.
The product has a moderate irritant effect on the mucous membranes of the eyes. HOW TO USE:
Hand hygiene:
3 ml of the product is applied to the hands and rubbed into the skin until dry for 30 seconds.
Treatment of the injection field, incl. at the vaccination site:
the skin is wiped with a sterile cotton swab or irrigated until completely moisturized, followed by holding for 30 seconds.
Preventive treatment of the feet, sanitary treatment of the skin:
With a generously moistened cotton swab, thoroughly treat each foot or area of skin with different cotton swabs or irrigate with the product until the skin is completely moisturized, holding time for at least 30 seconds.
Processing of gloves worn by personnel:
The outer surface of the gloves is thoroughly wiped with a sterile swab, generously moistened with the product. Processing time – at least 1 minute. Exposure - until the surface of the gloves is completely dry.
Small tools of simple configuration:
(in healthcare facilities, beauty salons, hairdressing salons, manicure and pedicure rooms, etc.) must be completely immersed in the product immediately after its use. The thickness of the layer of product over the products must be at least 1 cm. After the disinfection period of 5 minutes is completed, the products are removed from the container and washed with running drinking water for at least 1 minute.
As a prophylactic agent in the form of irrigation, rinsing and application - 5-10 ml of solution is applied to the surface of the skin or mucous membranes with an exposure of 1-3 minutes 2-3 times a day (on a tampon or by irrigation).
SPECIAL INSTRUCTIONS:
Use for external use only. Do not use the product after the expiration date. If the product accidentally gets into the stomach, it is recommended to drink several glasses of water with the addition of an adsorbent (for example, 10-15 crushed tablets of activated carbon per glass of water). Do not induce vomiting! If necessary, seek medical help.
RELEASE FORM:
The product is packaged in 25 ml, 50 ml, 100 ml in orange glass bottles. 25 ml, 50 ml, 100 ml, 200 ml, 500 ml, 1000 ml in high-pressure polyethylene bottles with a polymer nozzle, or with a special nozzle with a screw cap, or equipped with a spray pump and a protective polyethylene cap. 5 l each; 10 l; 20 liters in low-density polyethylene canisters (for hospital use).
STORAGE AND TRANSPORTATION CONDITIONS:
Store in tightly closed manufacturer's packaging at temperatures from 0 ºС to +30 °С; away from sources of heat and fire; Avoid storage in direct sunlight. No smoking! Store separately from medications, out of the reach of children. Transportation by any means of transport is allowed in accordance with the rules for the carriage of goods in force for this type of transport, at temperatures from 0 ° C to +30 ° C.
BEST BEFORE DATE:
The product's shelf life is 2 years in unopened manufacturer's packaging.
CONDITIONS FOR DISCHARGE FROM PHARMACIES:
Over the counter.
Why is Chlorhexidine better than peroxide?
- Hydrogen peroxide and Chlorhexidine are antiseptics. Medicines differ in their spectrum of effects and medicinal properties.
- Hydrogen peroxide, unlike Chlorhexidine, is available in only one dosage form, which is not very convenient.
Chlorkesidine suppositories are used for the treatment of gynecological and urological pathologies.
Both products disinfect wounds and abrasions well.
Chlorhexidine has a wider range of therapeutic effects; it is used to treat the hands of the surgeon and nurse before surgery, and it is also used in gynecology.
Chlorhexidine spray for external use alcohol 0.5% 100 ml bottle 1 pc. in Moscow
Externally.
During hygienic treatment of the hands of medical personnel
5 ml of the product is applied to the hands and rubbed into the skin for 2 minutes.
When treating the surgeon's hands
Before using the product, wash your hands thoroughly with warm running water and toilet soap for 2 minutes, and dry them with a sterile gauze cloth. Then apply the product to dry hands in 5 ml portions (at least 2 times) and rub it into the skin of the hands, keeping them moist for 3 minutes.
When treating the surgical field or elbow bends
donor skin is wiped twice successively with separate sterile gauze swabs, generously moistened with the product. Waiting time after finishing treatment is 2 minutes. On the eve of the operation, the patient takes a shower (bath) and changes his underwear. When treating the surgical field, the skin is wiped (in one direction) with a sterile swab moistened with the product. The holding time after completion of treatment is 1 minute. To disinfect small surfaces (including tables, equipment, armrests of chairs), the surfaces are wiped with a rag moistened with the product. The consumption rate for this treatment is 100 ml/m2.
Before disinfection of medical devices
remove visible dirt: from the outer surface - using cloth napkins moistened with water; the internal channels are washed with water using a brush or syringe in compliance with anti-epidemic measures (rubber gloves, an apron). Napkins, rinsing waters and containers for washing are disinfected by boiling or one of the disinfectants according to the regimens recommended for viral parenteral hepatitis (for tuberculosis - according to the regimens recommended for this infection), according to the current instructions and methodological documents. After removing the contamination, the products are completely immersed in the product solution, filling the cavities and channels with it. Detachable products are shipped disassembled. Containers with the solution should be tightly closed with lids to prevent the evaporation of alcohol and a decrease in its concentration.
For disinfection of products that have been previously washed from contamination,
the drug can be used repeatedly within 3 days (provided that the used product is stored in a tightly closed container to avoid changes in the alcohol concentration). When the first signs of a change in the appearance of the product appear (including the appearance of flakes, clouding), it should be replaced.