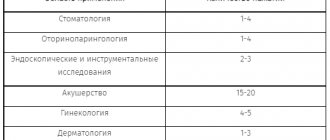
Nosological classification (ICD-10)
- B37.9 Candidiasis, unspecified
- J02.9 Acute pharyngitis, unspecified
- J03.9 Acute tonsillitis, unspecified (angina agranulocytic)
- J04.0 Acute laryngitis
- J37.0 Chronic laryngitis
- K05 Gingivitis and periodontal diseases
- K05.4 Periodontal disease
- K11.8 Other diseases of the salivary glands
- K12 Stomatitis and related lesions
- K14.0 Glossitis
- Z01.2 Dental examination
- Z100* CLASS XXII Surgical practice
When the remedy is ineffective
Often active advertising and the affordable price of a medicine lead to unpleasant phenomena, provoking self-medication.
The use of Tantum Verde for catarrh of the respiratory tract (tracheitis or bronchitis) is not justified. Here the remedy may even be harmful, spasming the bronchioles and causing suffocation.
Use this product with caution in children under 3 years of age.
In a number of instructions, not a single form of the drug is used for young children. However, the prescription of the drug is quite frequent even at this age, since it copes well with many problems in the mouth (in the absence of contraindications and the rules for using the drug are followed).
Until the age of 12, do not use an undiluted solution of the drug for rinsing.
During pregnancy and the lactation period, in some cases, topical use of the product in the form of a spray or solution is allowed.
Tantum Verde tablets are contraindicated for pregnant and nursing mothers.
Any use of Tantum Verde for nursing mothers and pregnant women is decided by the doctor.
Composition and release form
| Lozenges | 1 table |
| benzydamine hydrochloride | 3 mg |
| excipients: isomaltose; racementol; aspartame; citric acid monohydrate; mint flavor; lemon flavoring; quinoline yellow dye (E104); indigo carmine dye (E132) |
in a package there are 10 tablets, each of which is wrapped in waxed paper, placed in a wrapper made of two-layer aluminum foil; in a cardboard pack 2 packages (wrappers).
| Solution for topical use 0.15% | 100 ml |
| benzydamine hydrochloride | 0.15 g |
| excipients: ethanol 96%; glycerin (glycerol); methyl parahydroxybenzoate; menthol flavoring; saccharin; sodium bicarbonate; polysorbate 20; quinoline yellow dye 70% (E104); patented blue dye 85% (E131); purified water |
in bottles of 120 ml; complete with measuring cup; 1 set in a cardboard pack.
| Topical spray dosed 0.255 mg/dose | 100 ml |
| benzydamine hydrochloride | 0.15 g |
| excipients: ethanol 96%; glycerol; methyl parahydroxybenzoate; menthol flavoring (flavoring additive); saccharin; sodium bicarbonate; polysorbate 20; purified water |
in bottles equipped with a pump and a pressure device with a folding cannula of 30 ml; 1 bottle in a cardboard pack.
Tantum verde 3 mg 40 pcs. lozenges multi-flavor
pharmachologic effect
Non-steroidal anti-inflammatory drug.
Composition and release form Tantum verde 3 mg 40 pcs. lozenges multi-flavor
Lozenges - 1 tablet. benzydamine hydrochloride - 3 mg excipients: isomaltose; racementol; aspartame; citric acid monohydrate; mint flavor; lemon flavoring; quinoline yellow dye (E104); indigo carmine dye (E132) in a package of 10 tablets, each of which is wrapped in wax paper, placed in a double-layer aluminum foil wrapper; in a cardboard pack 2 packages (wrappers). Solution for topical use 0.15% - 100 ml benzydamine hydrochloride - 0.15 g excipients: ethanol 96%; glycerin (glycerol); methyl parahydroxybenzoate; menthol flavoring; saccharin; sodium bicarbonate; polysorbate 20; quinoline yellow dye 70% (E104); patented blue dye 85% (E131); purified water in 120 ml bottles; complete with measuring cup; 1 set in a cardboard pack. Spray for topical use dosed 0.255 mg/dose - 100 ml benzydamine hydrochloride - 0.15 g excipients: ethanol 96%; glycerol; methyl parahydroxybenzoate; menthol flavoring (flavoring additive); saccharin; sodium bicarbonate; polysorbate 20; purified water in 30 ml bottles with a dispenser; 1 bottle in a cardboard pack. Spray for topical use dosed 0.51 mg/dose (forte) - 100 ml benzydamine hydrochloride - 0.3 g excipients: glycerol; ethanol 96%; macrogol glyceryl hydroxystearate; menthol flavoring (flavoring additive); methyl parahydroxybenzoate; sodium saccharinate; purified water in bottles with a 15 ml dispenser; 1 bottle in a cardboard pack.
Description of the dosage form
Eucalyptus flavored lozenges: dark green translucent square tablets with a depression in the center with a characteristic eucalyptus odor.
Characteristic
NSAIDs belong to the indazole group.
Directions for use and doses
Inside, after eating. Adults (including elderly patients) and children over 6 years of age: 1 lozenge 3 times a day. Keep the tablets in your mouth until completely dissolved. Do not swallow. Do not chew. Do not exceed the recommended dose. For children from 6 to 12 years old, the drug is used under the supervision of an adult.
The duration of treatment should not exceed 7 days. If after treatment there is no improvement within 7 days or new symptoms appear, you should consult your doctor.
Use the drug only according to the method of use and in the doses indicated in the instructions. If necessary, please consult your doctor before using the medicine.
Pharmacodynamics
Benzidamine is a non-steroidal anti-inflammatory drug that belongs to the indazoles group. It has an anti-inflammatory and local analgesic effect, and has an antiseptic effect against a wide range of microorganisms. The mechanism of action of the drug is associated with the stabilization of cell membranes and inhibition of prostaglandin synthesis.
Benzidamine has an antibacterial and specific antimicrobial effect due to rapid penetration through the membranes of microorganisms with subsequent damage to cellular structures, disruption of metabolic processes and cell lysosomes.
Has antifungal effect against Candida albicans. Causes structural modifications of the cell wall of fungi and their metabolic chains, thus preventing their reproduction, which was the basis for the use of benzydamine for inflammatory processes in the oral cavity, including infectious etiology.
Pharmacokinetics
When applied topically, the drug is well absorbed through the mucous membranes and penetrates into inflamed tissues; it is found in the blood plasma in quantities insufficient to obtain systemic effects.
Excretion of the drug occurs mainly by the kidneys, in the form of inactive metabolites or conjugation products.
Indications for use Tantum verde 3 mg 40 pcs. lozenges multi-flavor
Symptomatic treatment of pain syndrome of inflammatory diseases of the oral cavity and ENT organs (of various etiologies):
- gingivitis, glossitis, stomatitis (including after radiation and chemotherapy);
- pharyngitis, laryngitis, tonsillitis;
- candidiasis of the oral mucosa (as part of combination therapy);
- calculous inflammation of the salivary glands;
- after surgical interventions and injuries (tonsillectomy, jaw fractures);
- after treatment and tooth extraction;
- periodontal disease.
For infectious and inflammatory diseases requiring systemic treatment, it is necessary to use Tantum® Verde as part of combination therapy.
Contraindications
- hypersensitivity to benzydamine or other components of the drug;
- fructose intolerance;
- children under 6 years of age;
- phenylketonuria, because contains aspartame (for mint or lemon flavored lozenges).
Carefully.
Hypersensitivity to acetylsalicylic acid or other non-steroidal anti-inflammatory drugs, bronchial asthma (including a history).
Use during pregnancy and breastfeeding Tantum® Verde should not be used during pregnancy or breastfeeding.
Application of Tantum verde 3 mg 40 pcs. multi-flavor lozenges during pregnancy and breastfeeding
Tantum® Verde should not be used during pregnancy and breastfeeding.
special instructions
When using the drug Tantum® Verde, hypersensitivity reactions may develop. In this case, it is recommended to stop treatment and consult a doctor to prescribe appropriate therapy.
In a limited number of patients, the presence of sores in the throat and mouth may indicate a more serious condition. If symptoms persist for more than 3 days, you should consult your doctor.
The use of Tantum® Verde is not recommended in patients with hypersensitivity to acetylsalicylic acid or other non-steroidal anti-inflammatory drugs.
The drug Tantum® Verde should be used with caution in patients with a history of bronchial asthma due to the possibility of developing bronchospasm while taking the drug.
The drug contains isomaltose and, therefore, is not recommended for patients with hereditary fructose intolerance.
The use of the drug in children is possible only under adult supervision to avoid swallowing the tablet.
Impact on the ability to drive vehicles and machinery
Does not affect the ability to drive vehicles and operate machinery.
Overdose
Currently, no cases of overdose of Tantum® Verde have been reported.
Symptoms: when using the drug in accordance with the instructions for use, an overdose is unlikely. If the drug is accidentally ingested, the following symptoms are possible: vomiting, abdominal cramps, anxiety, fear, hallucinations, convulsions, ataxia, fever, tachycardia, respiratory depression.
Treatment: symptomatic; empty the stomach by inducing vomiting or flush the stomach using a gastric tube (under medical supervision); provide medical supervision, supportive care and necessary hydration. No antidote is known.
Side effects Tantum verde 3 mg 40 pcs. lozenges multi-flavor
Classification of the frequency of side effects of the World Health Organization (WHO): very often - ≥1/10; often - from ≥ 1/100 to
Within each group, adverse effects are presented in order of decreasing severity.
Local reactions: rarely - dry mouth, burning sensation in the oral cavity; frequency unknown - feeling of numbness in the mouth.
Allergic reactions: uncommon – photosensitivity; rarely – hypersensitivity reactions, skin rash, itching; very rarely - angioedema, laryngospasm; frequency unknown - anaphylactic reactions.
If any of the side effects indicated in the instructions worsen, or any other side effects not listed in the instructions are noted, you should immediately inform your doctor.
Drug interactions
No interaction studies have been conducted with other drugs.
Pharmacodynamics
Benzidamine is a non-steroidal anti-inflammatory drug that belongs to the indazoles group. It has an anti-inflammatory and local analgesic effect, and has an antiseptic effect against a wide range of microorganisms.
The mechanism of action of the drug is associated with the stabilization of cell membranes and inhibition of PG synthesis.
Benzidamine has an antibacterial and specific antimicrobial effect due to rapid penetration through the membranes of microorganisms with subsequent damage to cellular structures, disruption of metabolic processes and cell lysis.
Has antifungal effect against Candida albicans. Causes structural modifications of the cell wall of fungi and the metabolic chains of mycetes, thus preventing their reproduction, which is the basis for the use of benzydamine for inflammatory processes in the oral cavity, including infectious etiology.
Antibacterial agents for the treatment of aphthous stomatitis
— After bacteriological analysis of the contents of aphthous stomatitis with determination of the causative agent of stomatitis, antibacterial ointments and gels are used.
- Actovegin;
- Holisal;
- Metrogil Denta;
- Wound healing;
— Sea buckthorn oil, rosehip oil, carotoline - these products contribute to better epithelization of defects in the oral mucosa.
— General treatment of aphthous stomatitis is aimed at relieving symptoms of intoxication, boosting immunity, and preventing complications.
— In the acute course of aphthous stomatitis at high temperatures, anti-inflammatory drugs are prescribed - ibuprofen, paracetamol.
— If stomatitis occurs against the background of a chronic disease, treat the underlying disease.
— In case of reduced immunity, the prescription of immunomodulators: immudol, ribomunil, thymalin.
Indications for Tantum® Verde
Inflammatory diseases of the oral cavity and ENT organs (of various etiologies):
gingivitis, glossitis, stomatitis (including after radiation and chemotherapy);
sore throat, pharyngitis, laryngitis, tonsillitis;
candidiasis (as part of combination therapy);
calculous inflammation of the salivary glands;
after surgical interventions and injuries (tonsillectomy, jaw fractures, etc.);
after treatment or tooth extraction;
periodontal disease.
For infectious and inflammatory diseases requiring systemic treatment, it is necessary to use Tantum® Verde as part of combination therapy.
Reasons contributing to the development of aphthous stomatitis
- Injuries to the oral cavity (mechanical, thermal, chemical).
- Inflammatory diseases of the teeth and nasopharynx: caries, periodontitis, gingivitis, tonsillitis, sinusitis, pharyngitis.
- Treatment with cytostatics and radiation therapy for cancer.
- Low quality dentures.
- Neglect of oral hygiene standards.
- AIDS.
- Pregnancy period and menopause in women due to hormonal imbalance.
- Diseases of the digestive system.
- Allergies of various origins (food, medication, microbial).
- Lack of complete proteins, vitamins B, C, folic acid, macroelements selenium, zinc in the diet.
- Diseases that cause depletion of the body (tuberculosis).
- Abuse of strong alcoholic drinks, smoking.
The symptoms of aphthous stomatitis in adults are determined by the pathogen, the general state of local and general immunity, and concomitant diseases.
- Acute aphthous stomatitis begins with fever, weakness, and headache. Regional lymph nodes are enlarged. Hyperemic round or oval spots appear on the oral mucosa, slightly rising above the surface. Then the areas of hyperemia become ulcerated. Erosions are painful, pain not only when chewing, but also at rest. Some erosions are covered with fibrin films. The plaque is tightly fused to the underlying tissues; when you try to remove it, the surface bleeds. Erosions are located singly on the mucous membrane of the lips, cheeks, and lateral surfaces of the tongue.
- Chronic aphthous stomatitis is formed either with inadequate treatment of the acute form of aphthous stomatitis, or with a significant decrease in general immunity. It is characterized by a cyclical course: periods of exacerbation are replaced by remission.
- Necrotic form of aphthous stomatitis. Not only the mucous membrane is affected, but also the submucosal layer. Tissue necrobiosis occurs. Aphthae are deep and their characteristic feature is painlessness. It is observed in debilitated patients with a sharp decrease in general immunity (AIDS, tuberculosis, cancer, blood diseases, severe infectious diseases). The healing period extends from 2 weeks to a month.
- Grandular - aphthae lie at the mouths of the minor salivary glands. Salivation is impaired. Relapse can occur after acute respiratory viral infections, with exacerbation of foci of chronic infection.
- The scarring form of aphthous stomatitis affects young people. The genetic factor of insufficiency of the secretory apparatus of the salivary glands plays a role. Aphthae capture not only the mucous membrane in the area of the terminal salivary sections, but also the underlying connective tissue. The ulcers are painful and reach 1.5 cm in diameter. Dissipated throughout the mucous membrane of the pharynx and anterior palatine arches. The healing process continues for up to 3 months, leaving scars.
- Deforming form of aphthous stomatitis. Due to the destruction of connective tissue and subsequent scar healing, the architecture of the oral cavity is disrupted.
Directions for use and doses
Locally. Lozenges: 1 tablet. 3–4 times a day. The tablet must be kept in the mouth until completely dissolved (for greater effect, preferably for as long as possible).
Solution for topical use: for gargling or mouthwash, use 15 ml of the drug (measuring cup included) 2-3 times a day.
An undiluted solution is used for rinsing during inflammatory processes; a diluted solution (15 ml of the drug and 15 ml of water mixed in a measuring cup) should be used daily for hygienic rinsing of the mouth and throat.
Topical spray dosed at 0.255 mg/dose: adults and elderly patients are prescribed 4-8 doses every 1.5-3 hours.
Children aged 6–12 years - 4 doses; 3-6 years - 1 dose for every 4 kg of body weight (maximum - 4 doses) every 1.5-3 hours.
Important benefits of use
Under the name "Tantum Verde" they produce several forms of a medicine made in Italy. These include:
- An undiluted 0.15% solution instantly provides relief from various catarrhal phenomena: soreness in the mucous membranes of the throat and mouth is eliminated, swelling of soft tissues is relieved, coughing is reduced, and purulent plugs from the tonsils are dissolved. In diluted form, it is used for the prevention of post-operative or post-traumatic complications (tooth extraction, tonsillectomy, jaw injuries).
- The tablet form is effective for acute or chronic catarrh of the mouth and throat, when the mucous membrane is swollen, painful and loose throughout. The tablets help relieve inflammation, soothe a sore throat and eliminate many of the patient’s sufferings.
- The spray is considered the ideal form of the product, penetrating deeply into the soft tissues of the throat for sore throat, stomatitis, pharyngitis or laryngitis. Ideal for children who do not yet know how to gargle or dissolve tablets. Allows you to clearly influence the source of inflammation.
Contraindications
It is important to carefully study the instructions before purchasing this medicine . Its use is unacceptable in the following cases:
- individual negative reaction to the drug;
- phenylketonuria;
- peptic ulcer in the acute stage:
- obstructive pulmonary diseases;
- heart failure;
- severe bronchial asthma.
Side effects
Rarely, the drug may cause the following undesirable effects:
- burning or numbness at the injection site;
- nausea or stomach pain;
- dry mouth mucous membranes;
- excess salivation;
- increased heart rate;
- dizziness or headache;
- Quincke's edema;
- diarrhea;
- noise in ears;
- allergic dermatoses;
- drowsiness;
- laryngospasm;
- confusion.
Such manifestations indicate individual intolerance to the drug.