1608
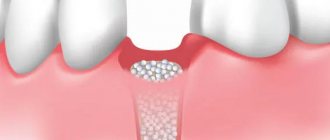
Barrier membranes are a modern surgical thin-sheet material designed to isolate surgical wounds filled with bone substitute for guided osteoregeneration.
Membranes are used to increase the efficiency of bone regeneration, accelerate wound healing, and eliminate bone resorption and postoperative complications.
Barrier films are used in dental surgery for various types of bone grafting, flap periodontal surgery, surgical correction of maxillodental anomalies, etc.
General overview
The American company Osteogenics Biomedical produces barrier membranes of various characteristics from natural and artificial materials.
Resorbable materials are made from natural raw materials - cattle tendons and porcine collagen.
The starting material for non-resorbable membranes is polytetrafluoroethylene. PTFE material can have a non-textured or textured surface, or be modified (hardened) with titanium.
Osteogenics Biomedical Barrier Membranes
| Resorbable | Size, mm | Non-resorbable | |||||
| Untextured | Size, mm | Microtextured | Size, mm | Titanium Reinforced | Size, mm | ||
| Cytoplast RTM Collagen | 30x40, 20x30, 15x20. | Cytoplast GBR-200 Singles | 12x24 | Cytoplast TXT-200 Singles | 12x24 | Cytoplast TI-250 ANTERIOR | 14x24, 12x24 |
| Cytoplast GBR-200 | 25x30 | Cytoplast TXT-200 | 25x30 | Cytoplast TI-250 BUCCAL | 17x25 | ||
| Cytoplast TI-250 POSTERIOR | 25x30, 20x25 | ||||||
| Cytoplast TI-250 XL | 30x40 | ||||||
Non-resorbable polytetrafluoroethylene (PTFE) dental membrane is an exclusive product not produced by anyone else other than Osteogenics Biomedical.
The material is intended for targeted regeneration of bone and gum tissue during surgical operations in dentistry.
Among the main purposes of Cytoplast GBR-200:
- providing primary wound coverage;
- preventing bone resorption;
- stimulating the growth of bone tissue from osteosubstitute;
- protecting the wound from pathogens;
- acceleration of wound healing;
- simplifying the work of surgeons.
Correction of malocclusions using Vector Tas microimplants and features of their use.
Come here if you are interested in the characteristics and purpose of Bio Oss bone material.
At this address https://www.vash-dentist.ru/implantatsiya/metodiki/temperaturyi-posle-zubov.html find out how long the temperature lasts after dental implantation with a positive outcome of the operation, and what indicators indicate the need to see a doctor.
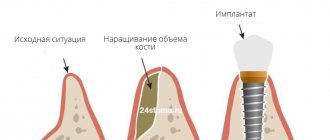
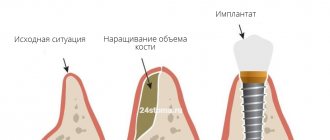
The success of patient rehabilitation after dental implantation is determined by the sufficient amount of bone tissue in the implantation site.
Filling bone defects with bone substitutes is the procedure of choice for bone maintenance, but ingrowth of connective tissue from the mucosa can disrupt the process of fusion of the bone substitute to the walls of the defects.
The use of a dental membrane as a barrier is indicated in the scientific literature as the most acceptable solution to this problem.
Guided bone regeneration (GBR) is a technique that is based on the concept of separating bone from soft tissue, that is, preventing apical migration of gingival epithelium and connective tissue within the defect with a membrane as a barrier. The use of a membrane promotes the proliferation of cells capable of regeneration and their differentiation into the desired tissue type.
For directed bone regeneration, five conditions are necessary:
- Use of an appropriate membrane;
- Achieving primary healing of soft tissues;
- Creation and maintenance of an area protected by a membrane;
- Adaptation and stabilization of the membrane with the surrounding bone;
- Quite a long healing period.
According to Hardwick et al., the main purpose of the membrane is to create suitable conditions under which the natural biological potential for functional regeneration is maximized.
Properties and types of membranes for dentistry
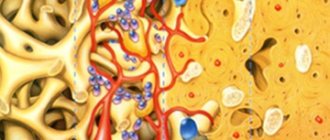
Creating and pre-preparing the site where the blood clot is located, preventing inflammation that may result from bacterial penetration, isolating the regeneration space from unwanted tissue, and ensuring mechanical stability and compactness of the organized coagulate are just a few of the factors for successful bone regeneration.
It is worth mentioning their role in preventing the penetration of epithelial cells into the bone material, as well as in enhancing the fixation of the bone substitute used.
Mandatory properties of a membrane for dentistry:
- Full biological compatibility;
- Barrier activity (prevention of soft tissue proliferation);
- Ensuring appropriate integration of surrounding tissues;
- The ability to preserve a site for the growth of new alveolar bone;
- Immunological inertia;
- Easy to use.
The membrane must resist chewing forces and reduce tissue tension.
In this case, the ability of the membrane to integrate with the preservation of surrounding tissues and effectively suppress epithelial migration is critical.
Based on clinical and histological studies of various barrier materials, no one has been shown to be ideal for every clinical situation. Each material has certain characteristics, advantages and limitations.
Depending on the response to their biological environment, membranes can be grouped as non-resorbable and resorbable.
Non-resorbable membranes for dentistry
retain structure and shape in the tissues, and to remove them it is necessary to perform another operation; this increases trauma, prolongs wound healing, and increases the cost and duration of the entire treatment.
Resorbable membranes
does not need to be removed after placement, which significantly improves practicality and optimizes treatment costs as well as the risk of surgical complications.
Due to the nature of resorbable membranes, it is impossible to accurately determine the duration of their destruction. The degradation process begins immediately after placement.
Data from the literature regarding the desired duration of membrane persistence in vivo indicate that it varies from 4 weeks to several months.
Due to biological degradation, resorbable membranes cause tissue reactions that can negatively affect wound healing and impair regeneration.
An ideal bioresorbable membrane for guided bone regeneration has the following characteristics:
- Biocompatibility;
- Lack of inflammatory response;
- Complete resorption, degradation and elimination;
- High technological effectiveness of the material;
- Optimal resorption time.
Such a dental membrane should be easy to handle, including delineating the shape of the defect and maintaining the required shape and configuration. It must be simply and reliably fixed, and be resistant to bacterial adhesion and colonization.
The most important requirement for membranes is the resorption time, which must correspond to the formation of new bone tissue.
Two materials are used to produce resorbable membranes: synthetic aliphatic polyester and collagen from various animal sources, including bovine tendon, bovine dermis, calf skin or porcine dermis.
Collagen membranes for dentistry
Collagen is an insoluble fibrous protein that is an important natural component of connective tissue.
Collagen's ability to stimulate adhesion, chemotaxis, and physiological degradation of progenitor cells along with its potential for degradation makes it an ideal material for creating membranes for dentistry.
There are at least 16 types of collagen found in interstitial tissues, bone matrix, cartilage, epithelial, blood vessels, and the vitreous body of the eye. Types I, II and III collagen make up 80–90% of the body's collagen.
Collagen products available on the market consist mainly of collagen types I and III.
Collagen is weakly immunogenic, activates hemostasis and can increase tissue thickness. During wound healing, clinically significant interaction of collagen with various cell types occurs.
Collagen is made from animal skin or by-products. The material is first isolated and purified with enzymes and chemicals, then processed in various ways.
The most common chemical modification of collagen forms cross-links, usually by exposure to an aldehyde, which reduces water absorption, affects meltability, degradation rate and stability.
This modified material is called cross-linked collagen.
Collagen membranes are degraded by macrophages and polymorphonuclear neutrophils, and the rate of uptake varies depending on the source and modifications of the collagen.
Resorption of the collagen membrane begins with the action of the enzyme collagenase (matrix metalloproteinase), which divides the collagen molecule at a certain position. The resulting parts are denatured and converted into gelatin, which is then broken down into amino acids by gelatinase and another proteinase.
During enzymatic digestion, it is incorporated into the flap to support the attachment of new connective tissue. This can result in an increased thickness of the flap, which protects and allows for further bone formation.
Collagen Cross-Linking: Pros and Cons
The stability of the structure, increased by cross-linking, slows down the degradation process.
The essence of this process is the creation of various mutual bonds between specific amino acids, as well as between amino acids and carboxylate groups, under the influence of chemical and physical agents.
Cross-linking of collagen is achieved by ultraviolet and gamma radiation, hexamethylene glutaraldehyde, diphenylphosphorylase and ribose.
This treatment reduces the in vivo rate of resorption of collagen material and improves mechanical properties. In the Western scientific world, there is still controversy over whether to use cross-linked or non-cross-linked collagen membranes for NRC.
Many studies have proven that cross-linking prolongs the biodegradation of the collagen membrane and that this shows a positive, although limited, effect on NRC in various types of experimental defect models. Other studies suggest that their use is associated with an initial foreign body reaction and impairs tissue integration and transmembrane vascularization.
Despite all the discrepancies, membrane vascularization has been shown to improve 2 weeks after submucosal implantation in a rat model. This is likely due to the fact that the initial hyperemia in the adjacent tissue drives angiogenesis towards the experimental membrane.
In 2006, Schwarz et al. investigated the angiogenesis model in natural and cross-linked collagen membranes because previous tests showed that vascularization in cross-linked membranes is weaker.
The researchers concluded that the rate of angiogenesis when using different types of membranes does not have statistically significant differences.
In studies conducted by researchers at the Belgrade Military Medical Academy, defects covered with cross-linked collagen membranes showed better levels of vascularization compared to non-cross-linked membranes and empty defects.
In 2012, Thoma et al. studied differences in cross-linked collagen using different degrees of cross-linking material in mouse soft tissue.
Histopathological and histomorphometric analyzes performed 3 and 6 months after the intervention showed that the degree of cross-linking of the material is inversely proportional to tissue integration, the degree of collagen biodegradation and the rate of vessel formation.
Collagen with the lowest degree of cross-linking exhibits better surrounding tissue integration, stability, and angiogenesis.
Despite several negative characteristics, many authors have suggested that the use of cross-linked collagen membranes has many advantages in targeted bone regeneration.
Efficiency of collagen membranes
Some periodontal microorganisms produce collagenase, an enzyme that can lead to premature membrane degradation. These are, in particular, Porphyromonas gingivalis and Bacteroides melaninogenicus.
Bacterial colonization can lead to early degradation of collagen membranes, compromising the procedure. Both cross-linked and non-cross-linked dental membranes are equally susceptible to lysis by bacterial proteases, although cross-linked membranes are somewhat more resistant to proteolysis.
Therapeutic concentrations of antibacterial agents, including chlorhexidine, minocycline, and doxycycline, partially inhibit enzymatic membrane degradation.
Collagen membranes vary in microarchitecture (the space between collagen molecules, fibers, bundles and layers) and cross-linking.
Microarchitecture and cross-linking determine membrane characteristics such as tensile strength, ease of use, flexibility, tissue integration, and biodegradation.
Membranes with a higher degree of cross-linking remain intact for a longer period of time. Research has shown that premature membrane resorption or removal can result in incomplete bone healing, so it is recommended that dental membranes used in NRC have a degradation period of 3 to 9 months for complete bone formation.
Biodegradation of collagen membranes
Rothamel et al. studied the biodegradation over time, tissue response, tissue integration and vascularization of commercially available collagen membranes as well as experimental products using rat models.
Histological and histometric studies were performed at 2, 4, 8, 16 and 24 weeks after membrane placement. It was concluded that cross-linked bovine and porcine type I and type III collagens prolong the biodegradation process but reduce tissue integration and vascularization.
In addition, when using these products in experiments, a foreign body reaction occurs, which is characteristic of cross-linked membranes.
This study highlights these differences between cross-linked and non-cross-linked membranes, proving that non-cross-linked membranes have better vascularization and tissue integration. The rate of absorption directly correlates with the degree of cross-linking - the higher the level of connections, the greater the rate of resorption.
In 2006 and 2008, Tal et al. clinically and histologically studied the duration of membrane barrier function and biointegration in sites that were treated with cross-linked and non-cross-linked collagen membranes.
Particular attention was paid to spontaneous perforations of the mucosa through barrier membranes. It was shown that the cross-linked membranes were more resistant to tissue degradation and that they maintained their integrity for a longer period.
Disadvantages of collagen membranes for dentistry
Compared to non-resorbable membranes, collagen membranes do not have the ability to maintain volume. The use of space-preserving bone graft tends to improve the outcome of guided bone regeneration.
Enlargement of the alveolar bone can be expected if the space under the collagen membrane is created and maintained strictly during the appropriate period while new bone is being formed.
It is therefore advisable to use materials that will provide support to prevent the barrier from collapsing due to the pressure generated by masticatory forces.
Today, dental membranes are commonly used with a variety of bone grafts and fillers. When bioresorbable membranes are used in this combination, the results of NRC are much more favorable, and comparable to non-resorbable membranes.
This is especially true when treating localized horizontal defects.
However, osteoplastic materials themselves are less effective than a combination of an auxiliary material and a barrier. The combination of membranes with bone material helps to achieve good results in the treatment of vertical defects of the alveolar process, since one of the main disadvantages of the collagen membrane is the inability to achieve the vertical height of the bone.
To solve this problem, the mentioned combination was used. The membranes in these cases require additional stabilization using mini-screws.
Effect of membrane thickness on bone regeneration
Until now, not enough studies have been published on the effect of resorbable membrane thickness on bone regeneration.
An attempt to use a thicker membrane was first published in 2005 by Busenlechner et al. The goal of their study was to question the ability of a slowly resorbing prototype three-layer membrane to regenerate bone tissue.
The model was a procedure used to increase the alveolar process after extraction of the first and second molars in the lower jaw of a monkey. Experimental animals were sacrificed after 9 months.
The study results support the use of a slowly absorbing three-layer membrane because the best bone regeneration achieved was achieved with this membrane and bone graft.
The membrane was made by adding a polylactide layer between the collagen layers to increase biodegradation time as well as barrier function.
Fragments of polylactides were detected in histological studies even 9 months after surgery.
Improving the design of the three-layer membrane can be an important step in improving the stability of the membrane at a certain impact rate.
The same prototype three-layer membrane was studied by von Arx et al. Their research looked at a prototype three-layer membrane in combination with various materials.
Differences were analyzed histopathologically and histomorphometrically after four and a half months. The prototype three-layer membrane combined with autograft demonstrated better bone regeneration.
In 2009, Kozlovsky et al. conducted a histological comparison of the biodegradation of Bio-Gide® membrane (a non-crosslinked collagen membrane) that was used in one or two layers with mechanical defects created on the jaws of rats.
Application of a second layer of Bio-Gide® membrane (double-layer technique) resulted in a significant increase in the residual amount of collagen.
There was also more barrier material left in the fabric, indicating that the barrier function of the membranes was longer lasting, and also that a single-layer membrane could not achieve similar barrier function over a longer period of time.
Thus, a two-layer membrane for dentistry significantly improves bone tissue regeneration and does not affect ossification. It should be noted that the second layer helps reduce micro-movements and increases the stability of the material.
Transmembrane vascularization was evident histologically as early as 4 weeks after implantation and became clearly visible across all layers of the membrane by 9 weeks after implantation.
Despite the difference in thickness of the double-membrane preparations, the same degree of degradation of 80% for both membranes was achieved after only 9 weeks.
Since transmembrane blood vessel formation is necessary for collagen resorption, it appears that the vascularization of the bilayer membrane was not impaired by its increased thickness.
It has been argued that increasing the density of cross-links between collagen molecules negatively affects biocompatibility, membrane integration, vascularization, and also inhibits the attachment and proliferation of fibroblasts and osteoblasts.
The use of a second layer of resorbable cross-linked membrane avoids these disadvantages, increasing the durability of the dental membrane.
The effectiveness of double-layer membranes in bone graft applications in terms of bone resorption was analyzed in studies in rabbits.
Bone blocks of the parietal bones were taken from one side and placed on the other, where they were covered with membranes. Histological and histomorphometric analyzes were performed 2, 4 and 6 months after surgery.
The results of the study show that the use of double membranes significantly reduces bone resorption of the graft compared to a single layer.
Another study, conducted by the Belgrade VMA, looked at the effects of collagen membranes of different thicknesses. The results showed that the best results were achieved with thicker dental membranes.
The results of a series of studies regarding membrane thickness show that thicker membranes, whether they are arranged in several layers or a single layer, exhibit a greater barrier function and remain in the tissue longer because they degrade slowly and promote better ossification of the bone defect.
Although the explanation for this finding remains elusive, it is suggested that the significant increase in membrane thickness and durability leads to an increase in angiogenesis and the cellular population of the collagen matrix, causing cell proliferation, differentiation and active ossification.
Collagen membranes of human origin
Particular attention should be paid to the absorbable collagen membrane for human dentistry.
The role of human resorbable demineralized membrane in guided bone regeneration and guided tissue regeneration is not well understood.
A number of European studies examining the influence of the thickness and origin of resorbable membranes on bone regeneration have yielded conflicting results.
Resorbable human demineralized membrane (RHDM) was obtained from cadaveric skull bone by a combination of physical and chemical methods, namely demineralization of cortical bone followed by removal of lipoproteins.
Experiments have shown that RHDM provides a higher rate of bone tissue regeneration compared to other membranes, especially when thicker membranes are used.
Combining growth factors
and membranes for dentistry
Recently, the inclusion of growth factors and differentiation factors in the membrane has attracted keen interest from researchers.
There is ample evidence that certain growth factors and similar mediators can influence the regeneration of many tissues, including bone regeneration.
An example is the development of combination membranes that can control the release of transforming growth factor (TGF-β).
Local delivery of a wide range of growth factors, such as platelet-derived growth factors (PDGFs) and bone morphogenetic proteins, which are osteoinductive growth factors, has already been successfully used in dentistry.
These substances have the ability to further stimulate cell migration, proliferation and differentiation. Numerous in vitro, animal and human studies have demonstrated the benefits of these factors in combination with collagen membranes.
Such innovations could lead to major changes in the results of guided bone regeneration without significant additional costs.
Conclusion
This article reviews the basic principles of using dental membranes in guided bone regeneration.
Significant progress has been made since the original non-resorbable polytetrafluoroethylene (e-PTFE) membrane was used.
Synthetic and natural biomaterials have been used in dental practice with great clinical success for more than 20 years; their mechanical properties and biodegradation rates are constantly being improved.
It is expected that the next generation of membranes will contain new functional molecules that improve the results of targeted bone regeneration.
Main characteristics
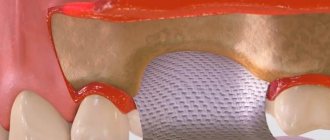
Cytoplast Regentex GBR 200 material is a dense polymer film 0.2 mm thick with a smooth surface.
The latter feature is a significant advantage over traditional resorbable membranes, the surface of which promotes retention and invasion by bacteria, causing rejection of the covering material and inflammation of the wound.
The smooth surface of GBR-200 “repels” pathogenic microorganisms, preventing infection and inflammation of the operating area.
The Cytoplast Regentex membrane has a microporous structure with a pore size of up to 1.26 microns, which does not allow bacteria to pass through the film, but at the same time is permeable to proteins.
The plastic-elastic consistency of the material allows it to be stretched and deformed, giving it the desired shape to adapt to the operating area.
Cytoplast has self-adhesive properties to the wound surface. As an additional measure of fixation, suturing the edges of the flap laid over Cytoplast can be used with light, tension-free sutures that do not affect the membrane itself.
The material comes in standard sizes 12x24 mm and 25x30 mm . From these blanks you can cut pieces of any size required in a specific clinical situation with scissors.
Composition and properties
The GBR-200 membrane consists of one PTFE (polytetrafluoroethylene (Teflon)) - a fluorocarbon compound that is resistant to almost any aggressive chemical agents.
Under human body conditions, PTFE is absolutely insoluble in substances and physiological fluids.
Physico-chemical and biological properties:
- chemical stability;
- extremely low allergenicity;
- self-adhesiveness to the wound;
- gas and light tightness;
- impermeability to bacteria;
- permeability to proteins.
Advantages and disadvantages
Cytoplast has a number of clinical and technological advantages compared to traditional suture materials, making it very popular among dental surgeons.
Advantages
- There is no need for primary flap closure of the wound fragment (no need to prepare and stretch the flap material).
- Non-germination of epithelium , due to the high barrier properties of the material.
- Elasticity (the membrane stretches in any direction, adapting to the wound).
- Stability of the structure (does not swell).
- There is no need to cover the membrane with anything from above , the film can remain completely exposed. To completely close the wound area, one Cytoplast GBR-200 is sufficient.
- Self-adhesive to the wound due to its special microstructure. There is no need to use complex fixing elements to attach the film to the bone; the edges of the wound do not need to be sutured.
- The absence of any negative consequences and chemical reactions when the material comes into contact with intraoral fluid.
- Fast wound healing.
- Preservation of the shape of the augmented alveolar bone tissue.
- Easy non-surgical removal of the membrane from the wound in 21-25 days. Removal is done through a small hole using a probe.
- Complete absence of any complications.
Flaws
Numerous tests have not revealed any significant drawbacks, which are often observed when using other types of membranes.
No rejection, inflammation, proliferation or other tissue reactions that negatively affected the healing process were noted.
The only drawback is the need for a dental surgeon to have practical skill in using the material , for which he has to undergo preliminary training.
Indications
The GBR-200 membrane is recommended for use in dentistry in all cases associated with directed bone regeneration:
- After tooth extraction to restore the bone tissue of the alveolar process.
- Before installing an implant if the bone volume is small.
- During or after installation of the implant - to close the wound.
- For augmentation in a wide variety of clinical cases.
- For peri-implantitis (inflammation of bone and soft tissues adjacent to the implant, their resorption and loss of the implant).
- For flap operations in the oral cavity, plastic surgery and gum recession.
- When restoring jaw proportions (for example, with a planar osteotomy, accompanied by replanting of bone tissue between separated fragments of the jaw bones).
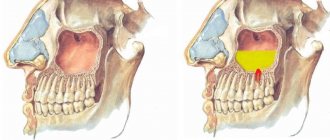
- During sinus lifting (Cytoplast is placed on the bottom of the maxillary sinus to eliminate the risk of penetration of the osteosubstitute into the sinus through perforations in the mucous membrane).
Let's discuss together the pros and cons of dental implantation in comparison with other prosthetic methods.
In this publication we will tell you whether you need to take antibiotics after dental implantation and why.
Follow the link https://www.vash-dentist.ru/implantatsiya/metodiki/rashozhdeniya-shvov-posle-zubov.html to find out when the sutures are removed after dental implantation.
What are barrier membranes?
The barrier membrane is one of the most modern and successful inventions in dental practice. Such designs are actively used in almost all surgical interventions. Barrier membranes for bone grafting during implantation can prevent atrophic phenomena in the alveolar process and contribute to the immediate successful fixation of artificial roots. The membrane affects the regenerative abilities of the bone, increasing them, does not allow osteoclasts that are dangerous to bone cells to enter, and minimizes the risk of penetration of pathogenic agents. Thanks to the barrier membrane, graft engraftment is accelerated, as is the overall treatment of the patient.
The membrane for bone grafting is a very thin and fairly elastic plate that is attached to the bone with titanium pins. In this way, the gums are separated from the bone material during the formation of natural jaw tissue.
Types of membranes
There are two types of barrier membranes that are widely used in dentistry:
- Resorbable. This structure gradually dissolves. It does not require separate removal, that is, additional surgical intervention, which means it causes less trauma.
- Non-resorbable. Membranes of this type do not dissolve on their own, which is why patients need an additional stage of treatment - removal of the structure. In most cases they are made of titanium. Such membranes are attached in the presence of a serious deficiency of bone tissue, filled with granules of osteogenic material. Once the alveolar bone and soft gum tissue have recovered, the barrier membrane is removed.
The dentist independently selects the optimal membrane for a specific clinical case. He must take into account that resorbable structures do not fix the grafts well enough, so they are used only for minor atrophies.
Technique of use
After filling the surgical wound with a bone substitute, Cytoplast GBR-200 is placed on top of it, overlapping the edges of the wound by 2-3 mm. In addition to self-adhesiveness, the film can be fixed to the wound using light, tension-free suturing of flaps placed on top of the Cytoplast.
The membrane is removed 21-25 days after the operation. The deadline is 28 days. Repeated surgery to remove Cytoplast is not required.
If a flap has been placed and sutured over the membrane, the material is extracted (pulled out) using a probe through a small hole . In most cases, anesthesia is not required (in extreme cases, topical anesthesia is used), bleeding, as a rule, does not occur.
After covering the surgical wound under the Cytoplast, bone and secondary epithelium begin to quickly form. Fusion of the flap with the newly formed epithelium usually occurs a month and a half after the operation.
In the video, watch the technique of using the Cytoplast membrane.
Diagnostics
To identify epiretinal fibrosis, it is necessary to examine the fundus, for which ophthalmoscopy is prescribed. Examination in transmitted light reveals its glimmer over the area of the yellow spot, reminiscent of reflection from cellophane film.
The epiretinal membrane in the initial stage is very thin, it may not be noticed, which complicates diagnosis. However, if there is a suspicion of its presence, an ultrasound examination is prescribed to confirm the diagnosis. It is also necessary when the optical media (vitreous body, lens, cornea) are opaque, which makes it difficult to examine the fundus using ophthalmoscopy.
Another diagnostic method for epiretinal fibrosis is optical coherence tomography, which helps to determine the size and structure of the membrane.
The degree of damage caused by the membrane can be determined by fluorescein angiography, which shows the extent of macular edema. How much the patient’s vision has deteriorated is determined using the Amsler grid test and visometry.
Fig. 2 Vitreoretinal surgeon in the operating room performing vitrectomy and macular peeling.
Clinical results
In order to determine the effectiveness of the GBR-200 membrane, the developer conducted studies of the material on a group of 10 patients in 3 clinical cases:
- tooth extraction;
- tooth extraction with implantation of a bone substitute;
- tooth extraction with implantation.
The following conditions for the operation were provided:
- the membrane was cut out using scissors according to the shape of the defect;
- when applied, the membrane overlapped the hole by 2-3 mm;
- adherence of the material to the wound was ensured by stretching and pressing with fingers and an instrument, taking into account the configuration of the alveolar process;
- The cytoplast remained on the wound for 21 days, after which it was carefully pulled off with tweezers.
Research results
- In no case of use of the GBR-200 material did an inflammatory process, cell proliferation or other negative tissue reactions occur.
- None of the membranes were rejected.
- All films were easily removed from the wound after 21 days without the use of anesthesia.
- On the wound surface, which was under the membrane, there was no resorption of surrounding tissues or epithelial growth. Only in some places along the edges of the wound were there migrating small areas of epithelium.
- The wound surface after removal of the Cytoplast was a newly formed bone structure.
Based on the results of the study, it was concluded that when Cytoplast GBR-200 was used for 3 weeks, a reliable barrier was created on the surface of the wound, which prevented the germination of epithelial tissue.
Reviews
The use of barrier membranes during surgical interventions is a new, modern technology that has not yet received the distribution it deserves.
If you have undergone surgery using Cytoplast barrier membranes, share your impressions of this material with other visitors to our site, leave your comment at the bottom of the page.
If you find an error, please select a piece of text and press Ctrl+Enter.
Tags: implantation, implantation methods
Did you like the article? stay tuned
Previous article
Should I save or remove a tooth in case of purulent periodontitis?
Next article
Universal trainer I-3 for the correction of malocclusions in children
Types of resorbable membranes
Resorbable membranes are called absorbable membranes, for the production of which the following materials are used.
- Polymers that can retain their properties for several months. Their advantages include a low risk of developing allergies and inflammation.
- Material of animal origin, characterized by high biocompatibility with human jaw tissue. Such membranes are used less frequently, as they can cause allergies.
Before installing barrier membranes, the patient undergoes a thorough examination to eliminate the risk of complications.