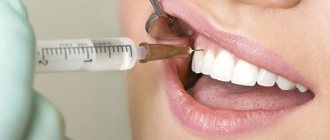
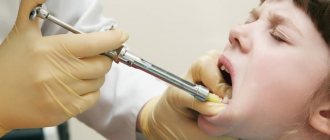
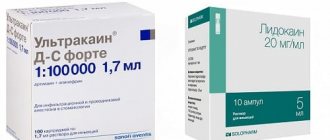
Anesthesia “Ultracaine”
There are times when it is impossible to do without anesthesia. Until recently, dentists used arsenic, which has a detrimental effect on the nerves. A new approach to dental pain relief has become possible thanks to ultracaine.
Advantages
Ultracaine is characterized by high efficiency and minimal side effects. It is twice as effective as lidocaine, and six times more effective than novocaine. Quickly passes into the tissue without affecting cardiovascular activity.
The active ingredients do not penetrate the placental barrier and do not enter breast milk, so the drug can be used during pregnancy and lactation.
Composition of the medicine
The active ingredients of the drug are hydrochlorides of artacaine and adrenaline (epinephrine).
Types of anesthetic
Ultracaine is available in three forms:
- DS Forte - due to its increased duration of action, it is ideal for operations and dental depulpation. Contraindicated in asthma, thyroid pathologies, hypertension, pregnancy and breastfeeding.
- DS - can be used during pregnancy, lactation, heart and vascular diseases. Contraindicated in asthma and thyroid pathologies.
- D - does not contain epinephrine, the duration of action is short - no more than half an hour. The drug is recommended for diseases of the thyroid gland, allergies, hypertension, asthma, and heart pathologies.
Mechanism of action
Ultracaine is used for local and regional anesthesia. It has a vasoconstrictor effect, thereby reducing pain.
The anesthetic effect appears almost instantly (in half a minute) and lasts for 1-5 hours. The drug blocks the transmission of nerve impulses, causing the patient to temporarily lose sensation.
It is eliminated from the body naturally within 6-12 hours.
The doctor selects the dosage and form of the medicine individually for each patient, taking into account age, weight, type of manipulations performed, and degree of complexity.
Indications and contraindications
Anesthetic is recommended for:
- filling, depulping and extraction of teeth;
- preparing the tooth for prosthetics;
- opening abscesses;
- inflammation of the maxillofacial area;
- surgical interventions.
Ultracaine is contraindicated for:
- individual intolerance to ingredients;
- anemia;
- heart failure;
- arterial hypertension;
- alcohol intoxication;
- less than 4 years of age.
Use with caution in case of bronchial asthma, arrhythmia, diabetes mellitus, hyperthyroidism.
Ultracaine should not be administered intravenously or injected into inflamed areas, or used simultaneously with inhibitors, beta-blockers and anticoagulants.
Ultracain D
Ultracaine D (active ingredient - articaine) is a drug for local anesthesia in dentistry. Inactivates voltage-gated sodium channels in neuronal membranes, which prevents the conduction of sensory nerve impulses in and around the injection site and causes local anesthesia. The drug does not contain adrenaline, which allows its use in cases where the latter is contraindicated. Ultracaine D begins to act 1-3 minutes after injection. The duration of action is about 20 minutes. The rapid hydrolytic breakdown of the active substance to a pharmacologically inactive metabolite prevents the development of systemic side effects and allows repeated injections at short intervals. Like other local anesthetics, articaine undergoes metabolic transformations by enzymes of the microsomal fraction. In addition to this, articaine is hydrolyzed in tissues and blood by nonspecific plasma esterases. Articainic acid, formed as a result of metabolic transformations, has no pharmacological activity or systemic toxicity. Elimination of the drug from the body is carried out mainly through urine. The dose of the drug is calculated based on the patient’s body weight. Before injection, it is recommended to perform an aspiration test to avoid the drug entering the blood vessel. Injections into inflamed gums should be avoided. Possible undesirable side reactions associated with the use of Ultracaine D: depressed mental state, manifested in lethargy, inactivity and silence (transitioning to loss of consciousness), breathing disorders, involuntary, rapid, rhythmic oscillatory movements of body parts caused by muscle contractions, vertigo, sensation of running goose bumps on the skin, decreased sensitivity to stimuli, transient visual impairment. These side effects are dose-dependent and, as a rule, go away on their own without any therapeutic intervention.
In some cases, if the injection technique is violated, the facial nerve is damaged. Among the nonspecific side effects of Ultracaine D are a painful sensation in the epigastric region, chest, oral cavity and pharynx, preceding vomiting (as well as vomiting itself), hypotension, decreased heart rate, increased blood flow to the skin, inflammation of the mucous membrane of the eye, nose, Quincke's edema, swelling at the injection site. Ultracaine D is not prescribed for severe dysfunction of the sinoatrial node, severe bradycardia, 2-3 degree atrioventricular block, acute decompensated myocardial dysfunction, severe hypotension, anemia, increased (over 1%) amount of methemoglobin in the blood, oxygen starvation, individual intolerance to articaine and others local anesthetics of the amide series. In pediatric practice, the drug is used starting from the age of 4. The use of Ultracain D during pregnancy is possible only under strict indications in cases where the potential benefit outweighs the possible harm to the mother and fetus. Lactation is not a contraindication to the use of the drug. To avoid infection, it is not recommended to reuse needles and syringes for injection. When using the drug, it is recommended to monitor the functional state of the heart, blood vessels, respiratory organs, and central nervous system. Eating is allowed after the local anesthetic effect has ended. The combined use of Ultracain D with drugs that inhibit the activity of the monoamine oxidase enzyme increases the risk of developing arterial hypotension. Local anesthetics potentiate the effects of CNS depressants. When using Ultracain D in people taking heparin or aspirin, bleeding is possible at the injection sites. Ultracaine D increases the duration of action and potentiates the effects of muscle relaxants.
Ultracaine D-S Forte
The drug is intended for use in the oral cavity and can only be injected into tissues where there is no inflammation. Do not inject into inflamed tissue.
The drug cannot be administered intravenously.
For anesthesia for uncomplicated extraction of teeth in the upper jaw in the absence of inflammation, it is usually sufficient to create a depot of the drug Ultracain® D-S (Ultracain® D-S forte) in the area of the transitional fold by introducing it into the submucosa from the vestibular side - 1.7 ml of the drug per tooth. In rare cases, an additional injection of 1 ml to 1.7 ml may be required to achieve complete anesthesia. In most cases, this eliminates the need for a painful palatal injection. When removing several adjacent teeth, the number of injections can usually be limited.
For anesthesia for incisions and sutures in the palate in order to create a palatal depot, about 0.1 ml of the drug is required for each injection.
In the case of removal of mandibular premolars in the absence of inflammation, mandibular anesthesia can be dispensed with, because Usually, infiltration anesthesia provided by injection of 1.7 ml of the drug per tooth is sufficient. If in this way it was not possible to achieve the desired effect, an additional injection of 1-1.7 ml of the drug should be performed into the submucosa in the area of the transitional fold of the mandible on the vestibular side. If in this case it was not possible to achieve complete anesthesia, it is necessary to conduct a conduction block of the mandibular nerve.
During surgical interventions, the dose of Ultracain® D-S forte, depending on the severity and duration of the intervention, is set individually.
When performing one treatment procedure, adults can be administered articaine in a dose of up to 7 mg per 1 kg of body weight. It was noted that patients tolerated doses up to 500 mg (corresponding to 12.5 ml of solution for injection) well.
In children over 4 years of age, the dose of the drug is selected depending on the age and body weight of the child; the dose should not exceed 5 mg of articaine per 1 kg of body weight.
In elderly patients and patients with severe renal and hepatic impairment, increased plasma concentrations of articaine may occur. In these patients, the drug should be used in the minimum dose necessary to achieve a sufficient depth of anesthesia.
In order to avoid accidental intravascular administration of the drug, an aspiration test should always be performed before its administration.
The injection pressure should correspond to the sensitivity of the tissue.
Overdose
Symptoms: the first manifestations of toxic effects are dizziness, motor agitation or stupor; possible bradycardia, a sharp decrease in blood pressure, breathing problems, muscle twitching, generalized convulsions, severe circulatory disorders, shock.
Treatment: at the first manifestation of symptoms of toxic action during drug administration, its administration should be stopped and the patient should be transferred to a horizontal position with the lower limbs raised. Airway patency should be ensured and hemodynamic parameters (heart rate and blood pressure) should be monitored. It is always recommended, even if the symptoms of intoxication seem mild, to insert an IV catheter so that, if necessary, you can immediately administer the necessary medications IV.
In case of breathing problems, depending on their severity, oxygen supply is recommended, and if there are indications for artificial respiration, endotracheal intubation and mechanical ventilation are recommended.
The administration of centrally acting analeptics is contraindicated.
Muscle twitching and generalized convulsions can be relieved with intravenous administration of short-acting or ultra-short-acting barbiturates. It is recommended to administer these drugs slowly, under constant medical supervision (risk of hemodynamic disorders and respiratory depression) and with simultaneous oxygen supply and monitoring of hemodynamic parameters.
Often, bradycardia or a sharp decrease in blood pressure can be eliminated by simply moving the patient to a horizontal position with the lower limbs elevated.
In case of severe circulatory disorders and shock, regardless of their cause, the drug should be discontinued and the patient should be transferred to a horizontal position with the lower limbs elevated. It is necessary to provide oxygen supply, intravenous administration of electrolyte solutions, corticosteroids (250-1000 mg methylprednisolone), if necessary, plasma substitutes, incl. albumin.
With the development of collapse and increased bradycardia, slow intravenous administration of a solution of epinephrine (0.0025-0.1 mg) under the control of heart rate and blood pressure is indicated. If it is necessary to administer in doses exceeding 0.1 mg, epinephrine should be administered by infusion, adjusting the rate of administration under the control of heart rate and blood pressure.
Severe tachycardias and tachyarrhythmias can be stopped by the administration of antiarrhythmic drugs, with the exception of cardio-nonselective beta-blockers.
Increased blood pressure in patients with arterial hypertension should be reduced, if necessary, with the help of vasodilators.
Ultracaine D-S, 100 pcs., 1.7 ml, 40 mg+5 μg/ml, solution for injection
The drug is intended for use in the oral cavity and can only be injected into tissues where there is no inflammation. Do not inject into inflamed tissue.
The drug cannot be administered intravenously.
For anesthesia for uncomplicated extraction of teeth in the upper jaw in the absence of inflammation, it is usually sufficient to create a depot of the drug Ultracain® D-S (Ultracain® D-S forte) in the area of the transitional fold by introducing it into the submucosa from the vestibular side - 1.7 ml of the drug per tooth. In rare cases, an additional injection of 1 ml to 1.7 ml may be required to achieve complete anesthesia. In most cases, this eliminates the need for a painful palatal injection. When removing several adjacent teeth, the number of injections can usually be limited.
For anesthesia for incisions and sutures in the palate in order to create a palatal depot, about 0.1 ml of the drug is required for each injection.
In the case of removal of mandibular premolars in the absence of inflammation, mandibular anesthesia can be dispensed with, because Usually, infiltration anesthesia provided by injection of 1.7 ml of the drug per tooth is sufficient. If in this way it was not possible to achieve the desired effect, an additional injection of 1-1.7 ml of the drug should be performed into the submucosa in the area of the transitional fold of the mandible on the vestibular side. If in this case it was not possible to achieve complete anesthesia, it is necessary to conduct a conduction block of the mandibular nerve.
When treating cavities and grinding teeth for crowns, with the exception of the lower molars, depending on the volume and duration of treatment, it is recommended to administer the drug Ultracain® D-S with a lower content of epinephrine into the area of the transitional fold on the vestibular side in a dose of 0.5-1.7 ml per tooth.
When performing one treatment procedure, adults can be administered articaine in a dose of up to 7 mg per 1 kg of body weight. It was noted that patients tolerated doses up to 500 mg (corresponding to 12.5 ml of solution for injection) well.
In children over 4 years of age, the dose of the drug is selected depending on the age and body weight of the child; the dose should not exceed 5 mg of articaine per 1 kg of body weight.
In elderly patients and patients with severe renal and hepatic impairment, increased plasma concentrations of articaine may occur. In these patients, the drug should be used in the minimum dose necessary to achieve a sufficient depth of anesthesia.
In order to avoid accidental intravascular administration of the drug, an aspiration test should always be performed before its administration.
The injection pressure should correspond to the sensitivity of the tissue.